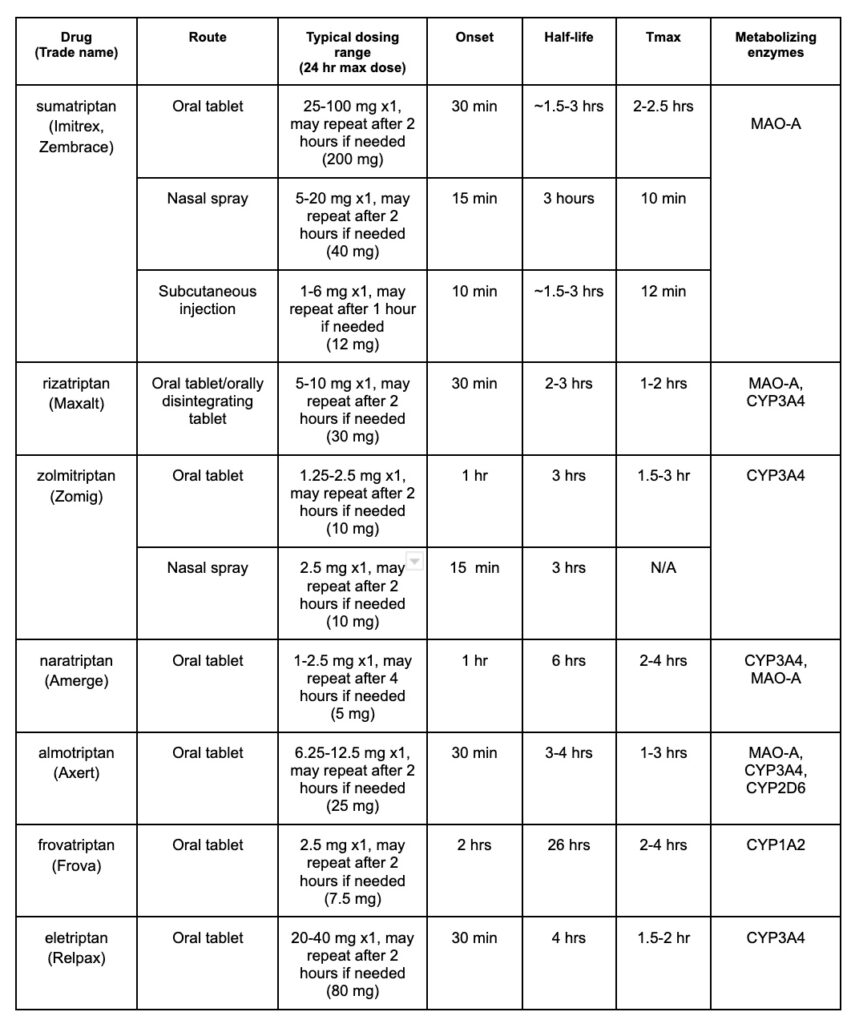
Acute migraine affects up to 26% of women, and up to 9% of men in the US. It is characterized by a moderate to severe headache that lasts for 4-72 hours and may be unilateral, cause nausea/vomiting, pulsating pain, or photophobia. First-line treatment options include acetaminophen, NSAIDs, and triptans. In this post, we will discuss triptans and we have provided a triptan comparison chart to help you remember some of the important differences between these agents for acute migraine.
Which Triptan to Choose?
As you can see, there are many triptan options to use when treating acute migraine, but how does one choose between them? The choice between these possibilities will mostly depend on patient preference and response. Studies have shown that there is not a significant difference when it comes to efficacy or adverse effects, although preference in route may guide the decision. After that point, the patient’s individual response to the medication can guide further decisions. If one triptan does not work well for a patient, trials have shown this does not predict response to others in the class, so the patient can try another triptan medication before reaching for other migraine treatment options.
Drug Interactions With Triptans
When comparing drug interactions with triptans, it is useful to know the mechanism of action and metabolism. Triptans work by binding to serotonergic receptors, specifically 5HT1B and 5HT1D, this causes vasoconstriction of the cranial arteries, which helps with migraine pain because it is due to the dilation of these arteries. Major metabolizing enzymes for many of the triptans include MAO-A and CYP3A4. There really isn’t a ton of variety between triptans and drug interactions (exception frovatriptan) so that is helpful in that we don’t need to memorize a bunch of different metabolic enzymes for each specific drug.
Due to major metabolizing enzymes, triptans may interact with other medications that are CYP3A4 inhibitors. Some medications to watch out for include ketoconazole, itraconazole, ritonavir, nelfinavir, and clarithromycin. Use of these medications within 72 hours of treatment with a triptan can lead to increased triptan concentrations in the blood as it will not be metabolized properly. On that same note, watch out for MAO inhibitors, which can cause that same effect. Drugs in this class include isocarboxazid, phenelzine, and tranylcypromine.
Due to their mechanism of action, another concern for interaction includes selective serotonin reuptake inhibitor (SSRI) or a selective serotonin-norepinephrine reuptake inhibitor (SNRI) medications such as citalopram, escitalopram, sertraline, fluoxetine, duloxetine, venlafaxine, and many others. Using these agents in combination with triptan medications may cause serotonin syndrome. It appears to be a relatively low risk, though it should still be considered and a risk-benefit discussion should occur in patients who will be taking both of these agents. I typically weigh the clinical risk based on the number of serotonergic drugs and the dosages employed. Some symptoms of serotonin syndrome these patients should look out for include confusion, muscle twitching or rigidity, agitation, and insomnia.
This is not an exhaustive list, so make sure to check the specific triptan your patient will be using to thoroughly assess for interactions.
Important Clinical Pearls
- Ideally, triptans should not be used for more than 9 days within 1 month. Overuse of these medications can cause “rebound headaches.”
- Triptans should be avoided in pregnant women, as data has shown that women who take triptans in the second and third trimester of pregnancy are at higher risk of developing uterine atony which can lead to increased blood loss after delivery.
- In pediatric patients, the only triptans that the FDA has approved for use include almotriptan and sumatriptan + naproxen (12-17 years), and rizatriptan (6-17 years). Despite this, some studies have shown nasal sumatriptan to be the most effective treatment in pediatrics, and although not FDA-approved, is recommended by some in pediatric patients who are 12 years or older.
If you’d like a Free PDF of the Triptan Comparison Chart, you can download that below. If you notice anything incorrect or that may have changed based on new evidence, please feel free to email me at [email protected]
Triptan Comparison Chart – Free Download
- 30 medication mistakes PDF
- 18+ Page Drug Interaction PDF
- 10 Commandments of Polypharmacy Webinar based on my experiences in clinical practice
Article written by Krista Olson, PharmD Candidate in collaboration with Eric Christianson, PharmD
References:
- Dahlöf CG. Infrequent or non-response to oral sumatriptan does not predict response to other triptans–review of four trials. Cephalalgia. 2006 Feb;26(2):98-106. doi: 10.1111/j.1468-2982.2005.01010.x. PMID: 16426262.
- Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001 Nov 17;358(9294):1668-75. doi: 10.1016/S0140-6736(01)06711-3. PMID: 11728541.
- Evans RW. Concomitant triptan and SSRI or SNRI use: what is the risk for serotonin syndrome? Headache. 2008 Apr;48(4):639-40. doi: 10.1111/j.1526-4610.2008.01067.x. PMID: 18377388.
- Wenzel RG, Tepper S, Korab WE, Freitag F. Serotonin syndrome risks when combining SSRI/SNRI drugs and triptans: is the FDA’s alert warranted? Ann Pharmacother. 2008 Nov;42(11):1692-6. doi: 10.1345/aph.1L260. Epub 2008 Oct 28. PMID: 18957623.
- Rolan PE. Drug interactions with triptans : which are clinically significant? CNS Drugs. 2012 Nov;26(11):949-57. doi: 10.1007/s40263-012-0002-5. PMID: 23018546.
- Gilmore B, Michael M. Treatment of acute migraine headache. Am Fam Physician. 2011;84(7):738]. Am Fam Physician. 2011;83(3):272.
- Ebell MH. Diagnosis of migraine headache. Am Fam Physician. 2006;74(12):2088.
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3-20.
- Dodick D, Brandes J, Elkind A, Mathew N, Rodichok L. Speed of onset, efficacy and tolerability of zolmitriptan nasal spray in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. CNS Drugs. 2005;19(2):125-36. doi: 10.2165/00023210-200519020-00003. PMID: 15697326.


0 Comments