As a consultant pharmacist, I always try to stay on top of trends to help nursing homes stay on top of guidelines and best practices. With the help of a student, we reviewed 50 LTC facility surveys and share the findings of the most common 2024 nursing home survey deficiencies. This helps me to identify trends and highlight educational opportunities. I was a little surprised by some of the data but not all of it. As a reminder, here are the important pharmacy-related F-tags that are part of the survey process (info from a previous post below).
- F-755; This deficiency can result from a few different concerns at a facility. In the data I have reviewed thus far, this deficiency is given for inadequate following of standard procedures in the storage and usage of medications. To give you a few examples, I’ve seen F-755 given for inadequate controls, not following policy and procedures surrounding counting narcotics, insulin beyond use dating, Advair beyond use dating, and orders that were never transcribed to the POS/MAR. I have a few case examples of drug diversion that can highlight the importance of counting narcotics and following appropriate policies and procedures.
- F-756; This is the classic “Drug Regimen Review” deficiency for a pharmacist who’s recommendations were not acted on or addressed. This can also be given if the pharmacist did not identify a problem, classically termed “irregularity”. Real examples of this deficiency include the pharmacist not recognizing appropriate target behaviors for the use of Seroquel (antipsychotic), parameters not in place in a resident on numerous analgesics, and the pharmacist questioning the use of PRN Ativan for >14 days without documentation from the provider, and the provider did not address the recommendation.
- F-757; Unnecessary medication is now under F-757. While this was historically found under the F-329, CMS elected to have psych medications have its own F-tag (I’ll get to that next). The examples I’ve seen cited thus far involve the use of pain medications; and duplicate analgesics without documentation of non-drug interventions tried and failed. F-757 and F-756 can be and have been “double dipped” with each other if the surveyor felt that the pharmacist did not catch this or if the pharmacist made a recommendation about the concern and it wasn’t acted on.
- F-758; Regimen must be free from unnecessary psychotropic medications; For those experienced in long term care, historically facilities would be tagged under F-329 for psychotropic issues or for any other medication that was deemed “unnecessary”. F-758 is PSYCH ONLY. The really big new addition here is the reassessment of as needed “PRN” psych medications that are used for greater than 14 days. Some examples of real deficiencies here are: haloperidol PRN for >14 days without documentation justifying longer term use, trazodone being used for sleep without any form of sleep monitoring being done, inadequate monitoring of Seroquel because an AIMS was not done, PRN Ativan >14 days without long term justification. F-758 is another category that can and will be double dipped with F-756 (pharmacist failure to notify). Hospice does not appear to be exempt from F-758.
- F-759; Free of medication error rates >5%. This one definitely isn’t cited very often because usually multiple mistakes have to be made in order to reach the 5% threshold. I have come across a couple examples where liquid dosages have been measure incorrectly as well as eye ointment not given by the correct procedure.
- F-760; This pharmacy services F-Tag requires that the resident be free of “significant” medication errors. I have not seen this one cited yet, but you could imagine that there is some subjectivity on what “significant” is. I’m not a huge fan of the CMS medication error tags F-759 and F-760 because I think there is a ton of subjectivity within these tags and it also attaches punitive action toward individuals making errors. In general, I think the best strategy to prevent these deficiencies is to have a good error reporting system and good procedures/processes in place to alter administration practices that are thought to be potentially harmful or not standard of care.
- F-761; Labeling and Storage of medications. You could make the argument that this does overlap a little with F-755. This deficiency will be given if medications are stored incorrectly or if there are issues with labeling. Examples here include; a nurse writing on a prescription label to change the directions, insulin in a fridge with no thermometer, expired insulin, disposal of fentanyl patches in a sharps container, and expired eye drops.
2024 Nursing Home Survey Deficiencies – By the Numbers
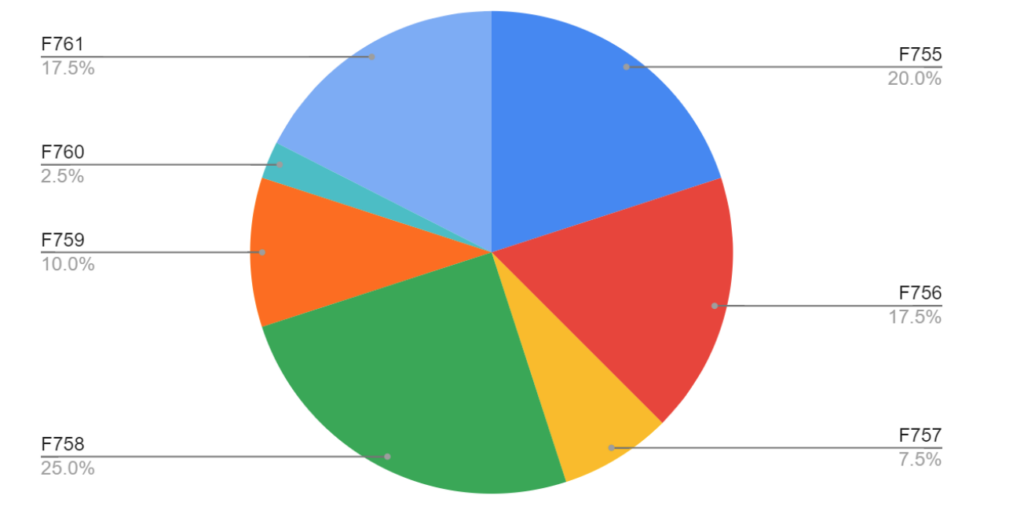
F758 and F755 have historically been the most frequently cited nursing home survey deficiencies. This remains true in 2024, as shown in the pie graph below. When we look at specific medications that are cited in these deficiencies, psychotropic agents take the cake with the greatest number of citations. Lorazepam, quetiapine, and trazodone were the three specific medications that had the highest incidence of nursing home survey deficiencies. This is likely due to the frequency of their use in long-term care facilities.
Examples of Pharmacy-Related LTC Deficiencies
I wanted to share a few specific examples of 2024 nursing home survey deficiencies.
F758: Free from Unnecessary Psychotropic Meds/PRN Use; No documentation of non-pharmacological measures attempted before administration of quetiapine.
F758: Based on the interview and record review, the facility failed to ensure that as-needed (PRN) psychoactive medications were not given without a rationale for continued use and indicated specific duration to prevent potentially unnecessary medications. There was no documentation for the continued need for a patient’s PRN lorazepam or trazodone.
F755: Pharmacy Srvcs/ Procedures/ Pharmacist/ Records; The facility failed to ensure a system for monitoring the plastic lock on the emergency kit (E-kit) that contained controlled substances to detect potential diversion at each change of shift. The kit contained the controlled medications of lorazepam 0.5 milligrams and morphine 20 mg/ml oral solution quantity.
The Surprising News – Vaccination Deficiencies F883
In our review of the 2024 nursing home survey deficiencies, I was pretty surprised by the number of deficiencies being cited on vaccines. F883 isn’t classified as a pharmacy-related deficiency, but I thought it was relevant to include. Facilities and staff should be aware of vaccine recommendations and stay up to date with current guidelines. This is something that surveyors are looking at and citing. This is particularly true of the Pneumococcal recommendations. For any facilities that I work with, I will be educating them to look out for this deficiency.
Example:
F883:Influenza and Pneumococcal Immunizations
The facility failed to offer and/or administer the most recent pneumococcal vaccine to 4 of 5 patients who had their charts reviewed.
If you are a pharmacist looking for more information on long-term care consulting and survey deficiencies, you can check out my popular educational course – Insider’s Guide to LTC Consulting.
This article was written by Eric Christianson, PharmD, BCGP, BCPS. Data and information gathering was done by Carly McDonald, PharmD Candidate.



0 Comments